Abstract
Background
Ruxolitinib (RUX) is a JAK1/JAK2 inhibitor approved for the treatment of hydroxyurea (HU)-resistant or intolerant patients with polycythemia vera (PV). Approval followed the results of the phase III RESPONSE trial, which demonstrated significant benefits of ruxolitinib in this population, including decreased phlebotomy requirements, reduction in splenomegaly, and amelioration of symptom burden. Although the results from the RESPONSE trial are positive in this selected patient population, no follow-up investigation has evaluated the real-world outcomes of patients with PV treated with RUX.
Methods
We conducted a retrospective case series of patients at a single-center tertiary-care institution who were prescribed RUX for the indication of PV between January 1st, 2013 and January 1st, 2017. Inclusion criteria included: 18 years of age or older, follow up at the study institution, and a diagnosis of PV without progression to myelofibrosis (MF) or other hematologic malignancy at time of initiation of RUX. Primary outcome measures included phlebotomy requirements, change in spleen size, and symptom burden (all assessed at 32 weeks after initiation of RUX), as well as the incidence of disease progression, and adverse events including infections, thromboses and cytopenias.
Results
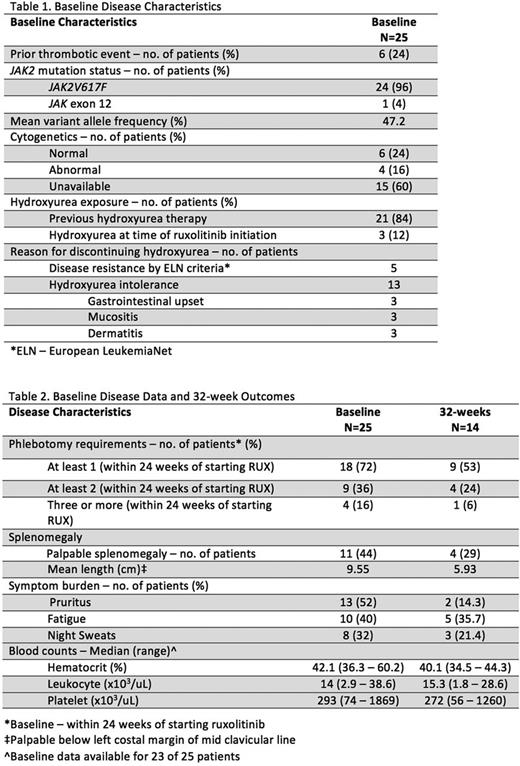
Baseline disease characteristics and history are summarized in Table 1. Twenty-five (16 men and 9 women) eligible patients were identified. The average age was 65.8 years (range, 51-89 years) at initiation of RUX. Patients started RUX, on average, 9.2 years after their diagnosis. Fourteen patients (56%) had follow up at or beyond 32 weeks of initiation (4 were lost to follow up, 3 started treatment before 32-week data could be captured and 4 discontinued RUX).
Primary outcomes relating to burden of disease are summarized in Table 2. Seven (50%) patients with 32-week follow-up data achieved phlebotomy freedom and four (29%) patients achieved a reduction in palpable splenomegaly of at least 50% (6 remained non-palpable, and the remaining 4 patients experienced reductions in palpable splenomegaly of less than 50%.
Four (16%) of 25 patients were deemed intolerant of RUX. Intolerance was reported as headache (2 patients), weight gain (1 patient), and dizziness/diarrhea (1 patient). The average time to discontinuation of the medication was 12.6 weeks. Excluding the 4 patients who discontinued RUX, patients were on the medication for an average of 70.4 weeks (range, 8.1-182 weeks). All patients who continued RUX past 32 weeks remained on the drug at their last known follow-up visit.
Three (13%) of 23 patients had leukopenia while on RUX, of which only 1 (4.3%) was grade III. Seven (30%) of 23 patients had thrombocytopenia while on RUX, of which only 1 (4.3%) was grade III. Eight (32%) of patients reported headaches while on RUX, 7 (28%) reported dizziness and 7 (28%) reported diarrhea. All reported adverse events were grade I/II. Two patients progressed to MF (on average 40.7 weeks after starting RUX). None progressed to AML or MDS. One patient, who had a previous thrombotic event, had a transient ischemic attack (grade I) while receiving RUX. No patient developed a secondary malignancy during the study period. Five (20%) patients had documented infections. Two were grade II (herpes recurrence and UTI), and one grade III (colitis), which were managed in clinic or the emergency department. Two infections, both pneumonia (grade III and V), required hospitalization.
One of these patients passed away from complications of a suspected lobar pneumonia 174.2 weeks after starting RUX. The patient, who had JAK2V617F-negative/exon 12-positive disease, presented with fever and cough, and was managed in the intensive care unit with broad-spectrum antibiotics before transitioning to hospice care.
Conclusions
This is the first study to evaluate the real-world effects of RUX for the treatment of PV. Patients in our case series, most of whom had failed therapy with HU, experienced an overall reduction in phlebotomy requirement, splenomegaly, and symptom burden after the initiation of RUX. The intolerance rate to RUX in this case series was comparable to, or lower than, the reported rates for HU. Although over half of patients reported an adverse effect of RUX, less than 20% discontinued the drug for this reason. The results of this case series are largely congruous with the positive results of the original RESPONSE trial.
Mascarenhas: Novartis: Other: DSMB member , Research Funding; Promedior: Research Funding; Merck: Research Funding; CTI Biopharma: Research Funding; Janssen: Research Funding; Incyte: Other: Clinical Trial Steering Committee , Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal